A 0.01 M solution of acetic acid is 1.34 % ionized (degree of dissociation = 0.0134 ) at 298 . What is the ionization constant of acetic acid.
What is the pH of 0.1 m of acetic solution, if acetic acid is a weak acid with Ka2 1.86 × 10-5? - Quora
The pH of an acetic acid solution is 3.26. What is the concentration of acetic acid and what is the percent of acid that's ionized? - Quora
Calculate amount of acetic acid which should be added in 300ml of 0.1 Molar of acetic acid solution (ka=10^ 5) to double its (i) alpha (II) pH
How to calculate the pH of 0.010 molarity acetic acid solution, if its dissociation constant is 1.8*10^-5 - Quora
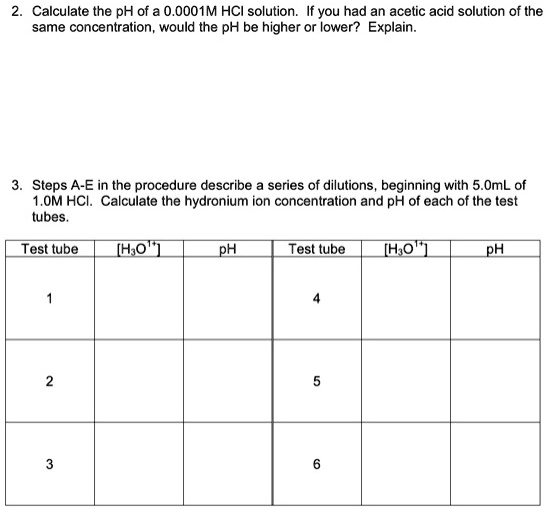
SOLVED: Calculate the pH of 0.0OO1M HCI solution If you had an acetic acid solution of the same concentration, would the pH be higher Or lower? Explain. Steps A-E in the procedure
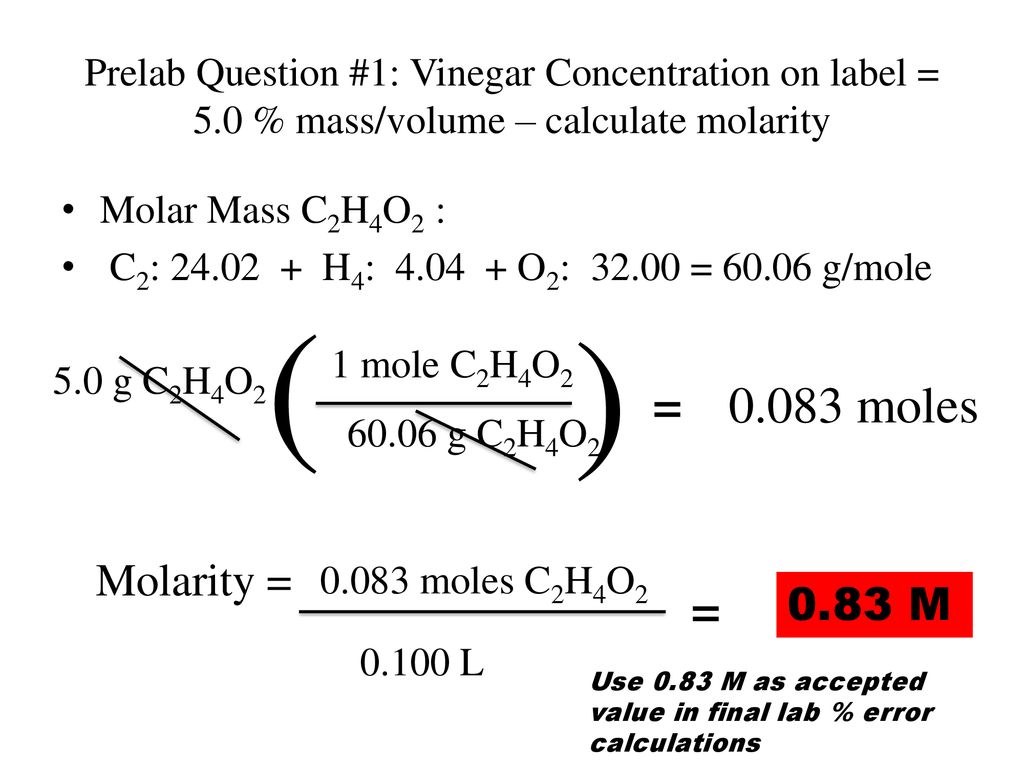
Titration What is the concentration in moles/liter of a vinegar (solution of acetic acid in water)? - ppt download

The degree of ionization of a 0.1 M bromoacetic acid solution is 0.132 . Calculate the pH of the solution and the pKa of bromoacetic acid.