Implementation of a reference-scaled average bioequivalence approach for highly variable generic drug products of agomelatine in Chinese subjects - ScienceDirect
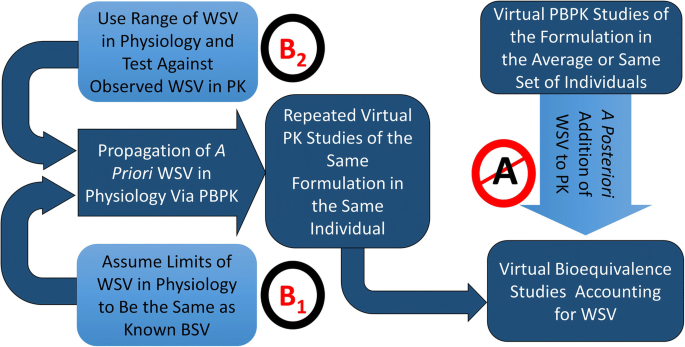
Proof of Concept in Assignment of Within-Subject Variability During Virtual Bioequivalence Studies: Propagation of Intra-Subject Variation in Gastrointestinal Physiology Using Physiologically Based Pharmacokinetic Modeling | SpringerLink

In Vitro Predictive Dissolution Test Should Be Developed and Recommended as a Bioequivalence Standard for the Immediate-Release Solid Oral Dosage Forms of the Highly Variable Mycophenolate Mofetil | Molecular Pharmaceutics

Adjusted Indirect Treatment Comparison of the Bioavailability of WHO‐Prequalified First‐Line Generic Antituberculosis Medicines - Gwaza - 2014 - Clinical Pharmacology & Therapeutics - Wiley Online Library
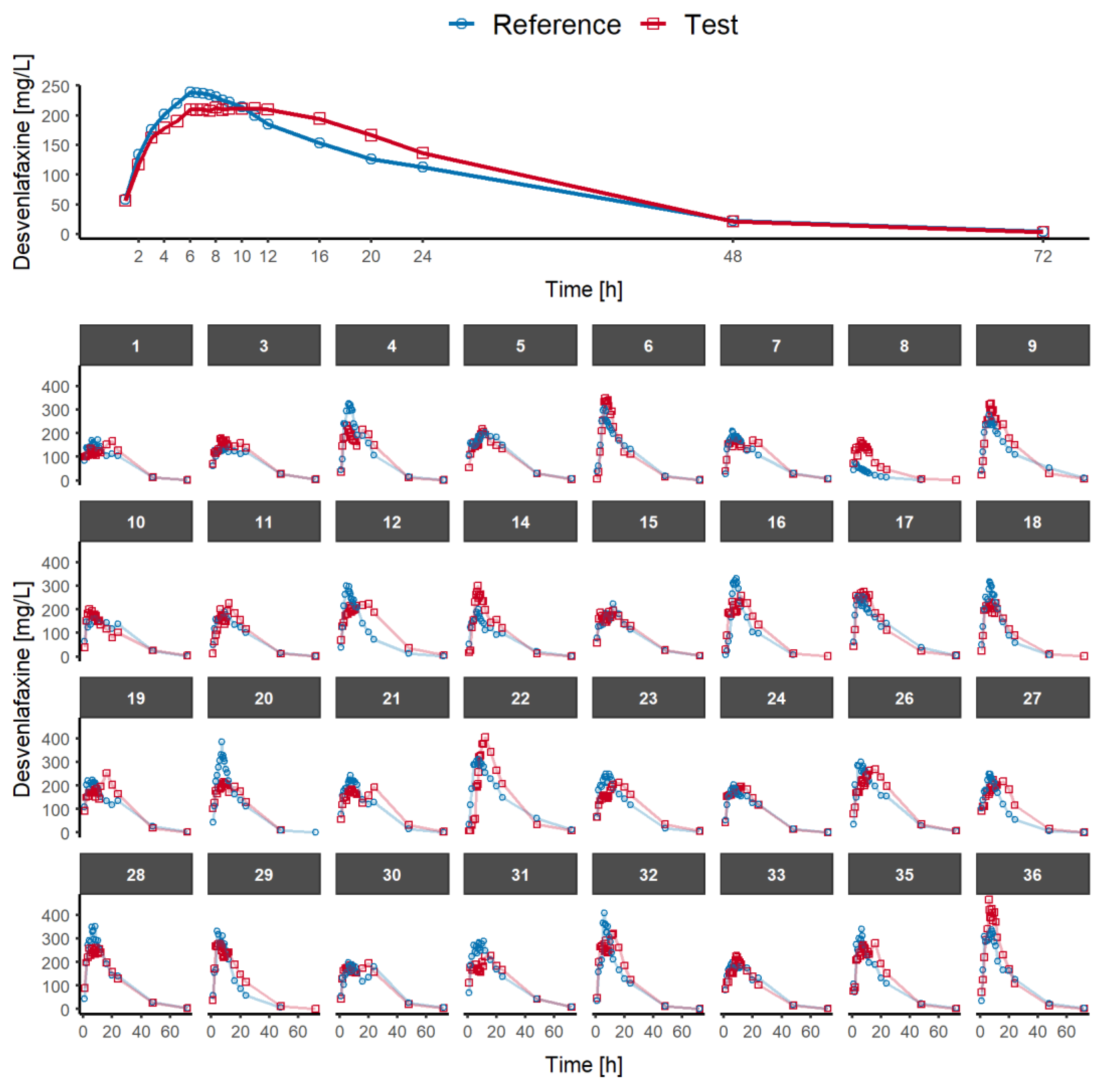
Pharmaceutics | Free Full-Text | Alternative Pharmacokinetic Metrics in Single-Dose Studies to Ensure Bioequivalence of Prolonged-Release Products at Steady State—A Case Study

Equivalence tests for ratio of means in bioequivalence studies under crossover design - Yingdong He, Yuhao Deng, Chong You, Xiao-Hua Zhou, 2022
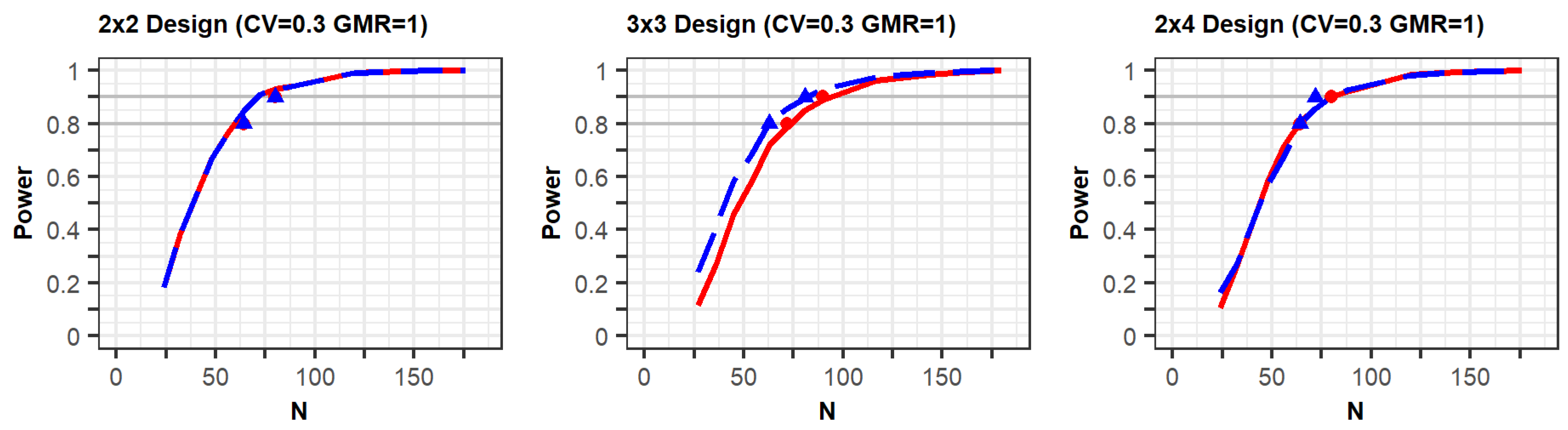
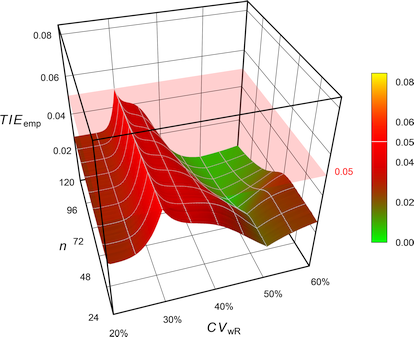
Pharmaceuticals | Free Full-Text | Model-Based Approach for Designing an Efficient Bioequivalence Study for Highly Variable Drugs

PDF) Between-Batch Pharmacokinetic Variability Inflates Type I Error Rate in Conventional Bioequivalence Trials: A Randomized Advair Diskus® Clinical Trial

Percent of studies passing bioequivalence (BE) (power curves); average... | Download Scientific Diagram

The 80–125% BE limits are represented along the x-axis as two " goal... | Download Scientific Diagram

Between-Batch Bioequivalence (BBE): a Statistical Test to Evaluate In Vitro Bioequivalence Considering the Between-Batch Variability | SpringerLink

PDF) Statistical Design Based on 90 % Confidence Intervals Analysis of Bioequivalence Studies of Sustained Release Capsules of Metoprolol Tartrate
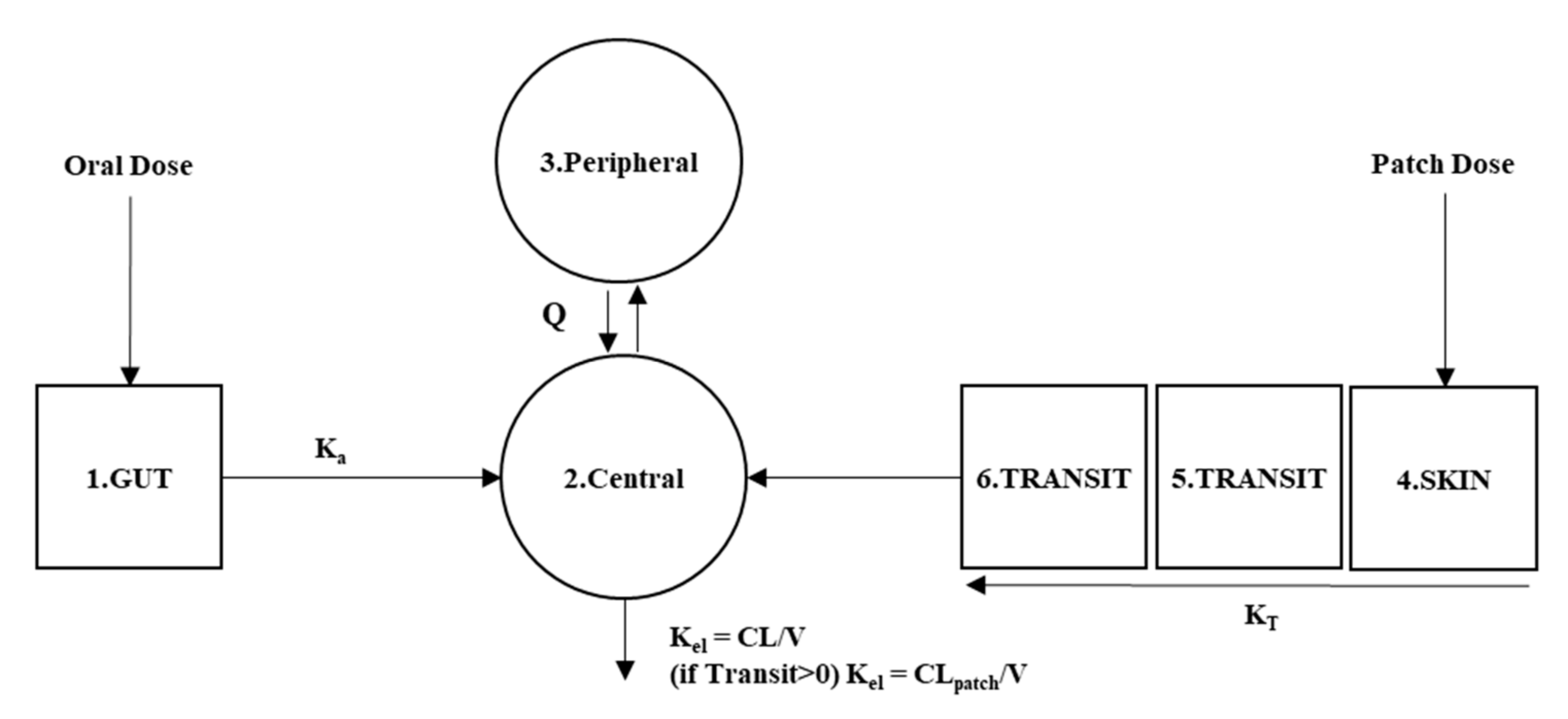
Pharmaceutics | Free Full-Text | Model-Based Equivalent Dose Optimization to Develop New Donepezil Patch Formulation

Between-Batch Bioequivalence (BBE): a Statistical Test to Evaluate In Vitro Bioequivalence Considering the Between-Batch Variability | SpringerLink

Sample size determination in bioequivalence studies using statistical assurance - Ring - 2019 - British Journal of Clinical Pharmacology - Wiley Online Library

PDF) Pooled bioequivalence study database from Turkey: characterization of adverse events and determination of split points based on Gini Index as a promising method
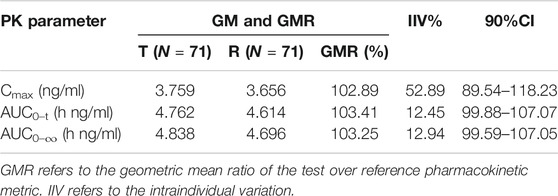
Frontiers | Pharmacokinetics and Bioequivalence of Rasagiline Tablets in Chinese Healthy Subjects Under Fasting and Fed Conditions: An Open, Randomized, Single-Dose, Double-Cycle, Two-Sequence, Crossover Trial

Pharmaceuticals | Free Full-Text | Model-Based Approach for Designing an Efficient Bioequivalence Study for Highly Variable Drugs