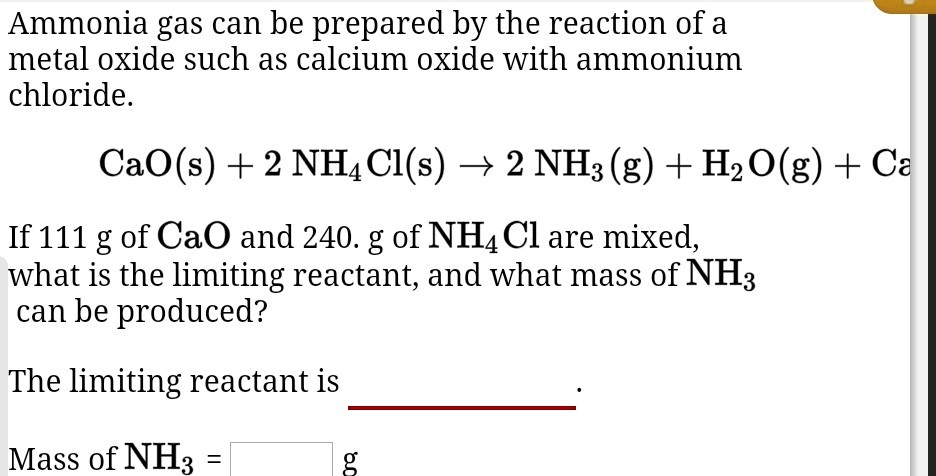
![SOLVED: CaCO3(heat) = CaO + CO2 Determine the ∆G for the reaction of CaCO3(s) forming solid calcium oxide at 35° C. The concentrations of [CaCO3], [CaO], and [CO2] are 0.15 M, 0.25 SOLVED: CaCO3(heat) = CaO + CO2 Determine the ∆G for the reaction of CaCO3(s) forming solid calcium oxide at 35° C. The concentrations of [CaCO3], [CaO], and [CO2] are 0.15 M, 0.25](https://cdn.numerade.com/ask_previews/c929f6cd-30ac-4b5d-81c0-5164da820942_large.jpg)
SOLVED: CaCO3(heat) = CaO + CO2 Determine the ∆G for the reaction of CaCO3(s) forming solid calcium oxide at 35° C. The concentrations of [CaCO3], [CaO], and [CO2] are 0.15 M, 0.25

Question Video: Calculating the Mass of Calcium Carbonate Required to Produce a Given Mass of Calcium Oxide | Nagwa
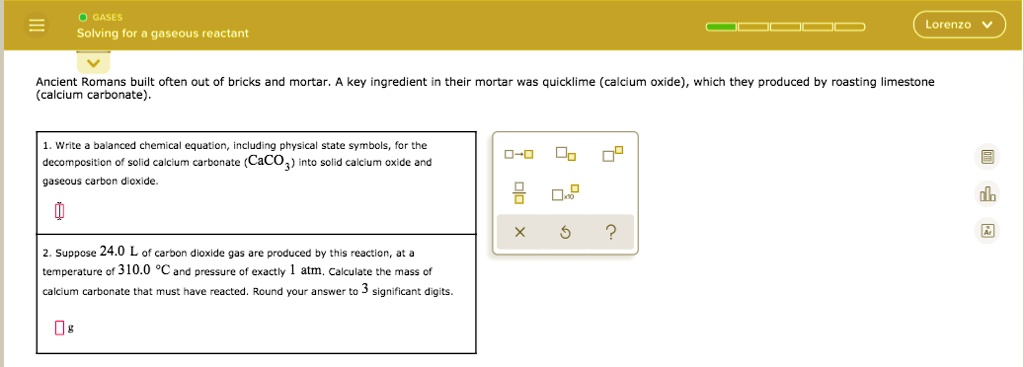
SOLVED: GASES Solving Ior gjascous reuctant Lorenzo Ancient Romans built often out of bricks and mortar, key ingredient their mortar was quicklime (calcium oxide) , "hich they produced bY roasting limestone (calcium