Activating copper oxide for stable electrocatalytic ammonia oxidation reaction via in-situ introducing oxygen vacancies | SpringerLink

Carbon monoxide oxidation on copper manganese oxides prepared by selective etching with ammonia - ScienceDirect

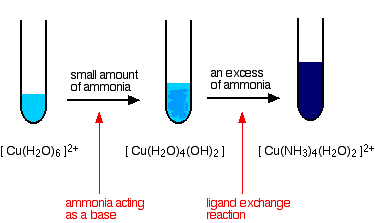
When dry ammonia gas is passed over hot copper (II) Oxide, a shinny brown residue and a colourless droplets are formed. Explain these two observations
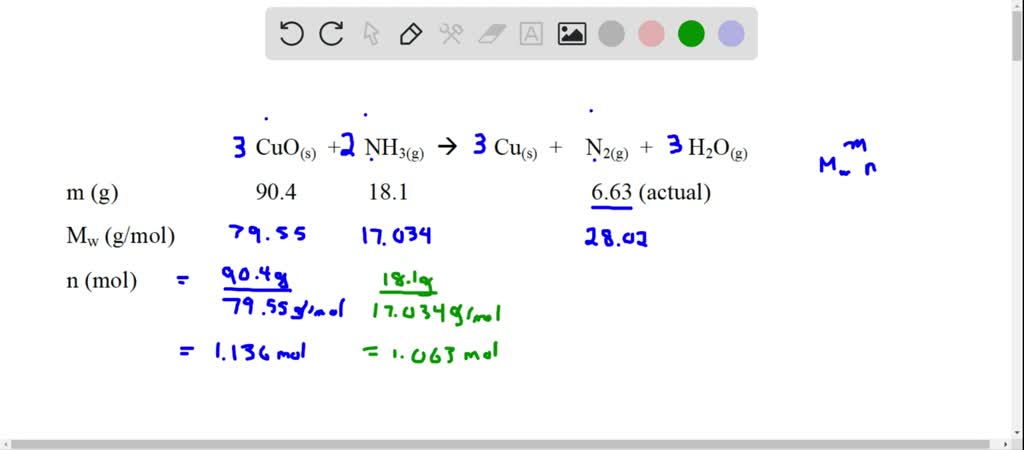
SOLVED: Gaseous ammonia, NH3, and copper(II) oxide, CuO, react together to form gaseous nitrogen (N2), solid copper, and water vapor. If a sample containing 17.9 g of NH3is reacted with 90.0 g

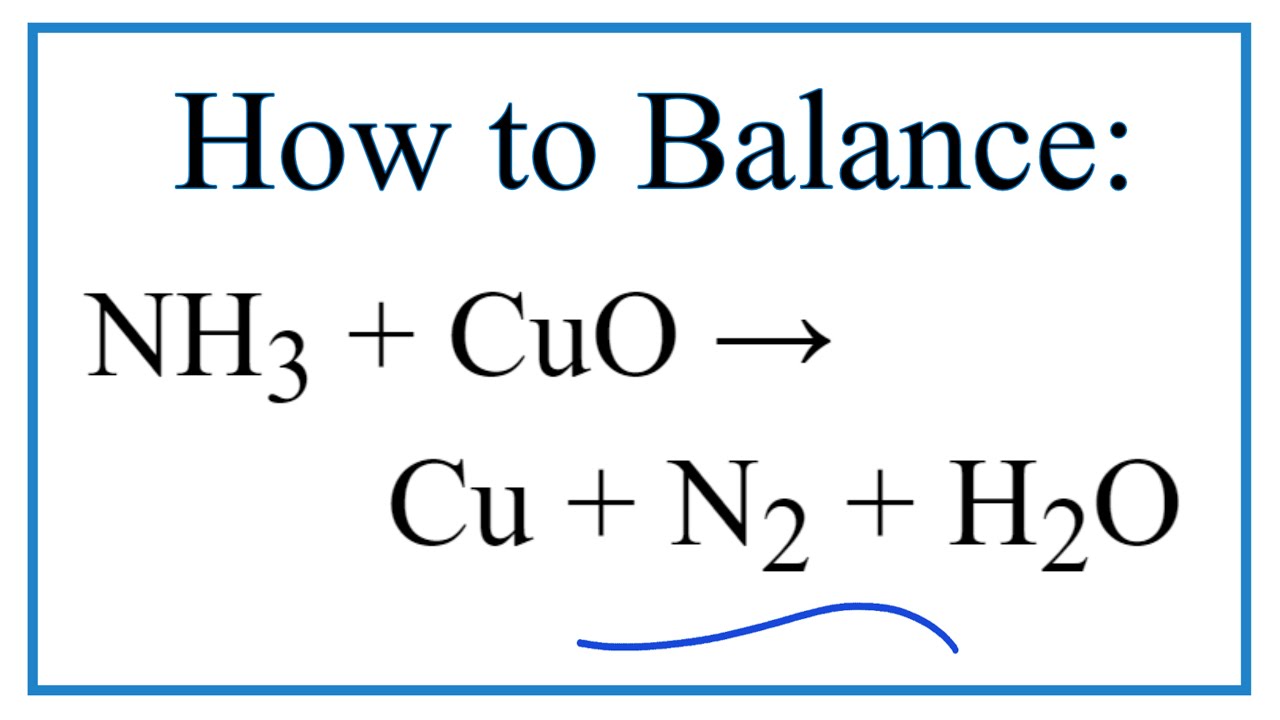
When ammonia is passed over heated copper oxide, the metallic coper is obtained. The reaction shows - YouTube

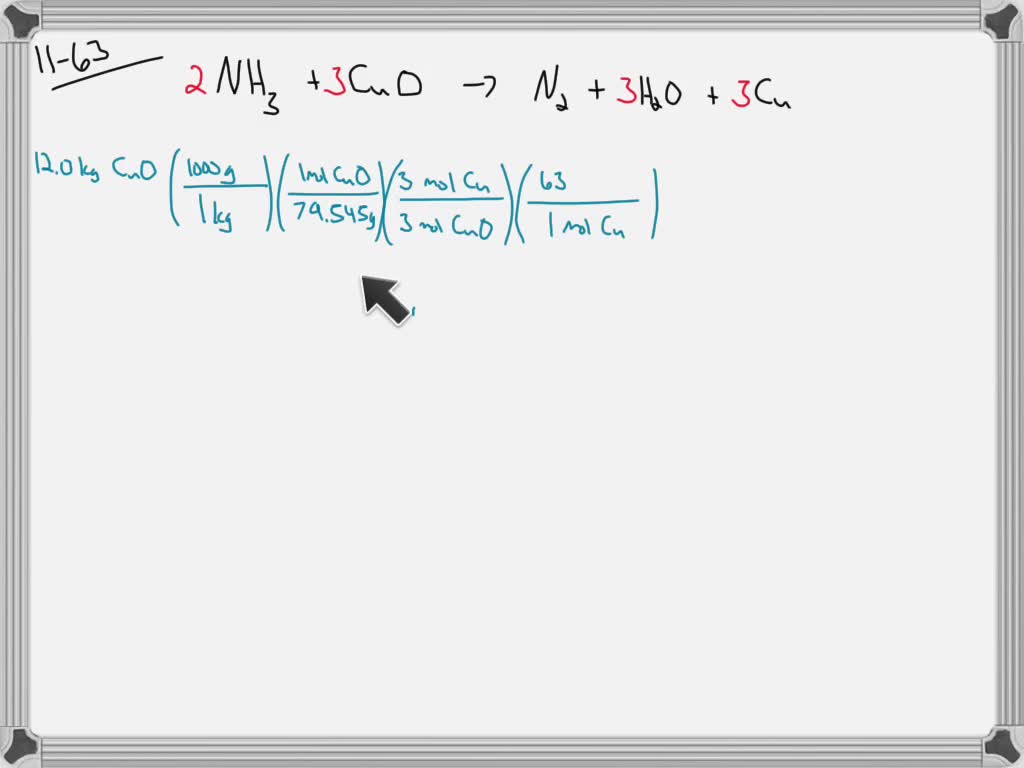
Given that the relative molecular mass of copper oxide is 80 , what volume of ammonia measured at STP is required to completely reduce 120 g of copper oxide? The equation for

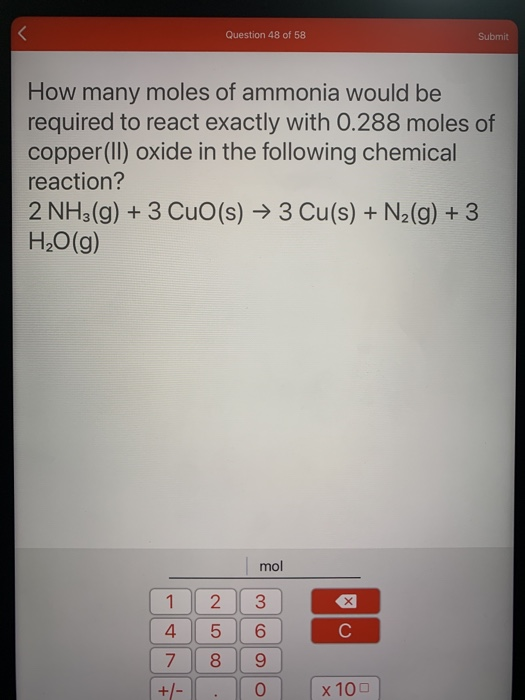
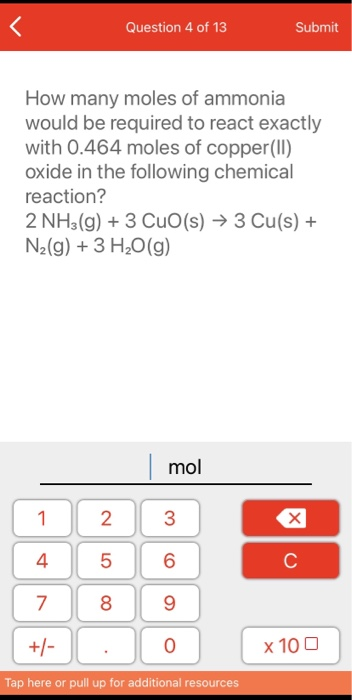
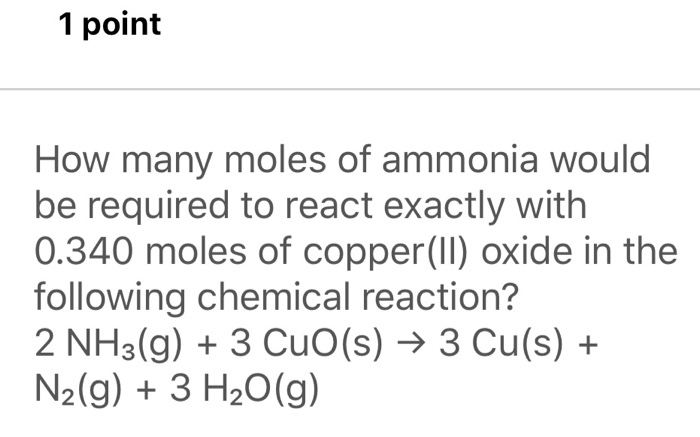
SOLVED: How many moles of nitrogen gas would be produced if 3.40 moles of copper(II) oxide were reacted with excess ammonia in the following chemical reaction? 2 NHz(g) + 3 CuO (s) -

Electrocatalytic, Homogeneous Ammonia Oxidation in Water to Nitrate and Nitrite with a Copper Complex | Journal of the American Chemical Society
SOLVED: 1) Ammonia and copper (II) oxide react according to the following equation: 2NH3 + 3 CuO → N2 + 3 Cu + 3 H2O if 57.0 g of ammonia are combined
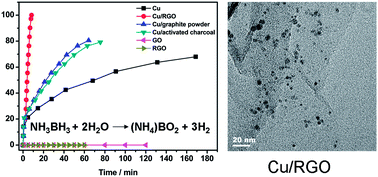
Facile in situ synthesis of copper nanoparticles supported on reduced graphene oxide for hydrolytic dehydrogenation of ammonia borane - RSC Advances (RSC Publishing)