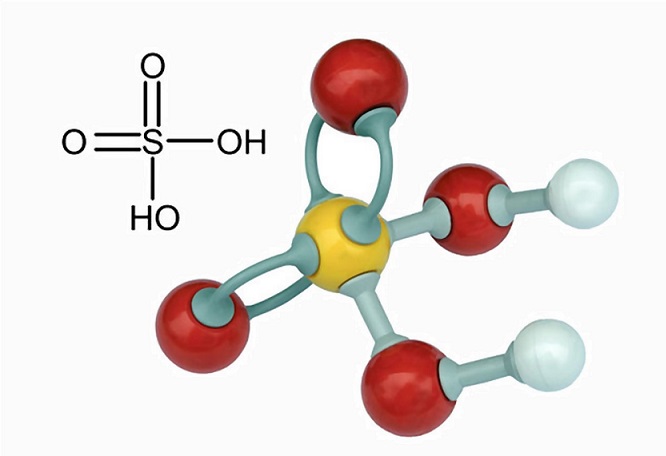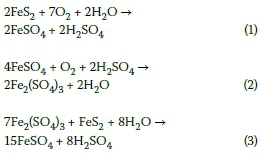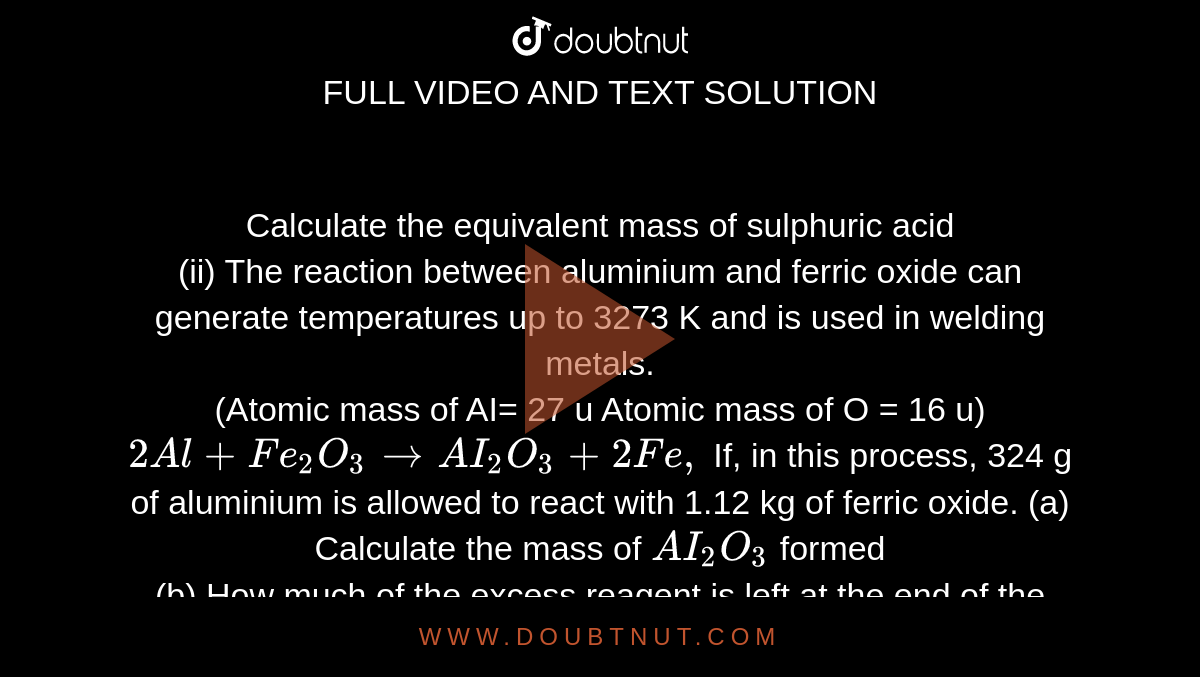
Calculate the equivalent mass of sulphuric acid (ii) The reaction between aluminium and ferric oxide can generate temperatures up to 3273 K and is used in welding metals. (Atomic mass of AI=

THE RICO-ARGENTINE CO. SULPHURIC ACID MILL. ALTHOUGH THE MILL HAS BEEN CLOSED FOR SIX YEARS, WATER SEEPING FROM OLD MINE SHAFTS POLLUTES NEARBY STREAMS WITH IRON OXIDE Stock Photo - Alamy

7.2 Making Salts What is made when acids and metals react? What is made when alkalis and metals react? 27 August 2015 Bonneville salt flats in America. - ppt download

How to Balance Fe + H2SO4 = FeSO4 + Fe2(SO4)3 + H2O + SO2 (Iron + Concentrated Sulfuric acid) - YouTube

Sulfuric Acid LO: Outline uses and reactions involving Sulfuric Acid Starter: What is an acid? - ppt download

Fe2O3+H2SO4=Fe2(SO4)3+H2O Balanced Equation|| Balanced equation for Iron iii oxide and Sulfuric acid - YouTube

XLIV.—The system ferric oxide—sulphuric acid—water - Journal of the Chemical Society, Transactions (RSC Publishing)

balance the equation :- ferric oxide reacts with sulphuric acid and give ferric sulphate and water. - Brainly.in

The process of pyrite weathering in a deep metal mine. Four general... | Download Scientific Diagram