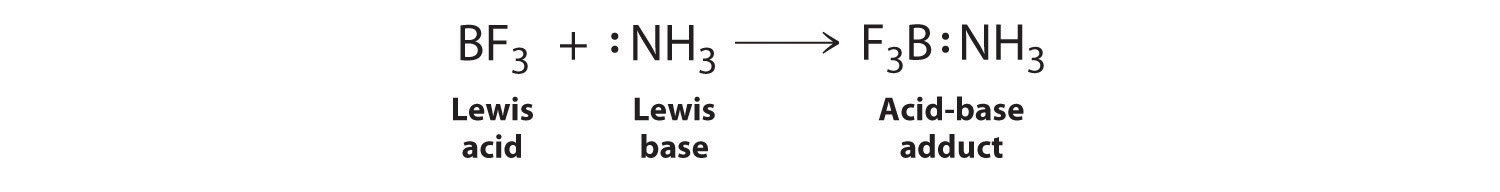
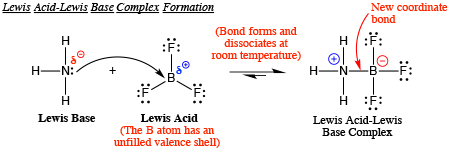
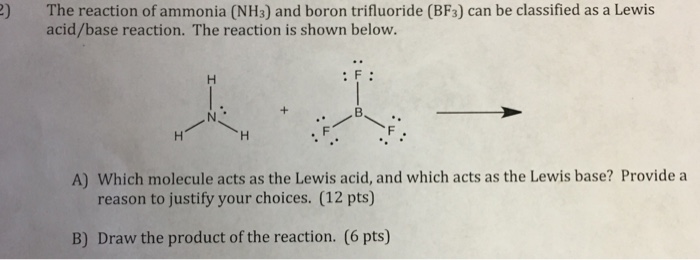
Why does boron trifluoride (BF3) act as a Lewis base and ammonia (NH3) acts as a Lewis acid? - Quora

Classify the Substances as Lewis Acids or Lewis Bases | Identifying Lewis Acids and Bases Practice - YouTube
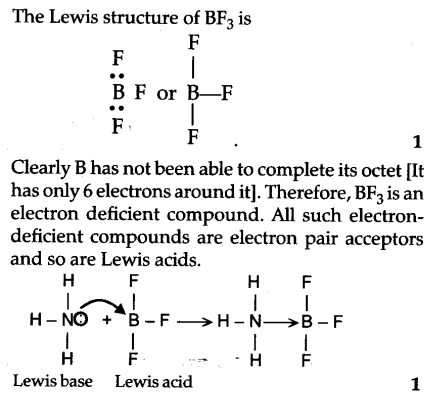
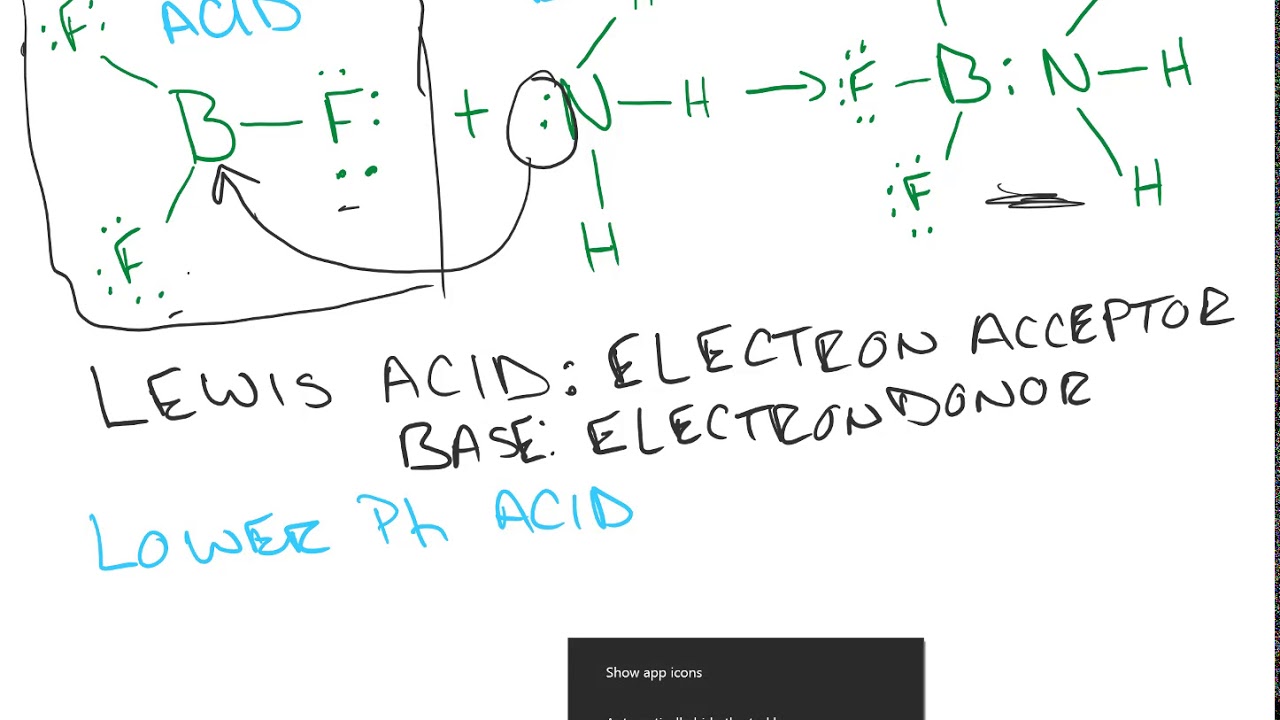
Explain : (A) BF3 is a Lewis acid, (B) NH3 is a Lewis base. - Sarthaks eConnect | Largest Online Education Community

Question Video: Identifying the Species That Is Not a Lewis Acid in a Set of Chemical Formulas | Nagwa

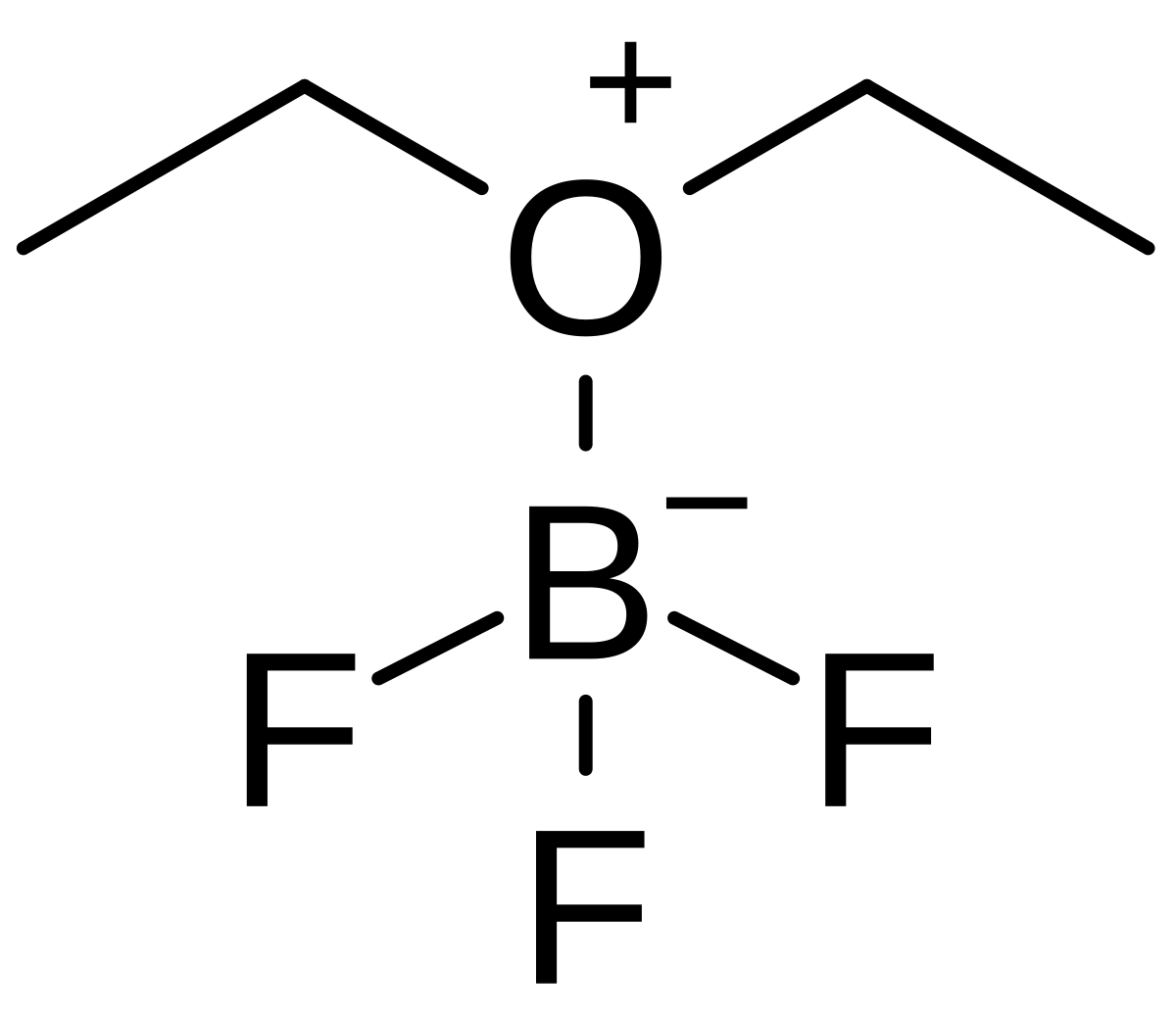
CH_3CH_2OCH_2CH_3 is best classified as a ______. A) Bronsted-Lowry acid. B) Lewis acid. C) Bronsted-Lowry base. D) Lewis base. E) Both C & D. | Homework.Study.com