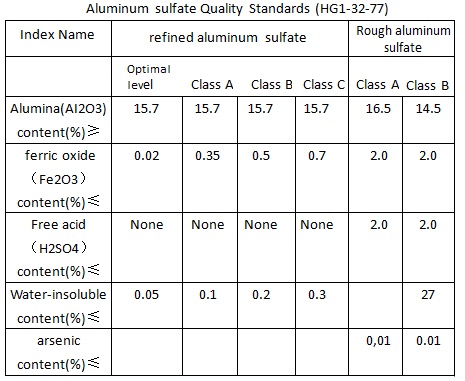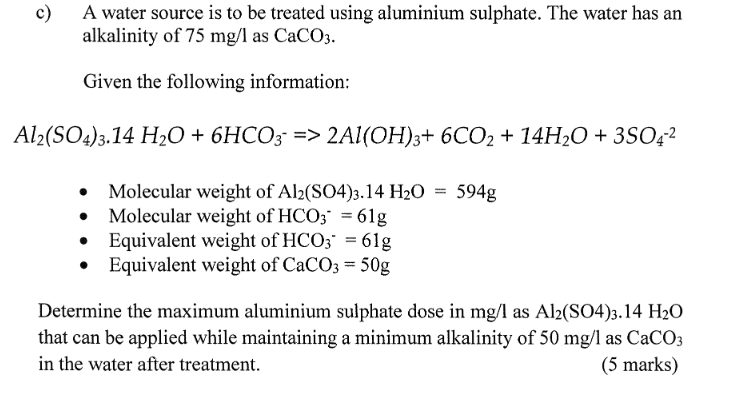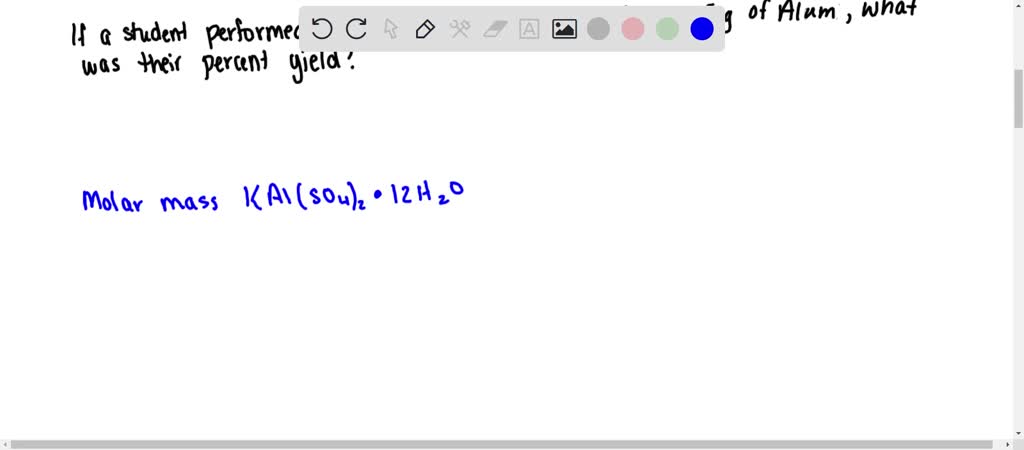
SOLVED: After preparing a sample of alum, K2SO4-Al2(SO4)3-24H2O, a student determined its purity gravimetrically in a sample of 1,2931 g, the aluminum precipitated as: Al(OH)3. The precipitate was collected by filtration, washed,
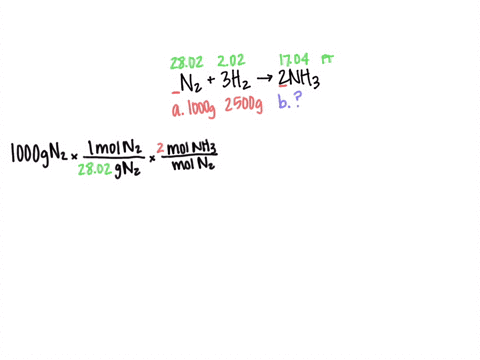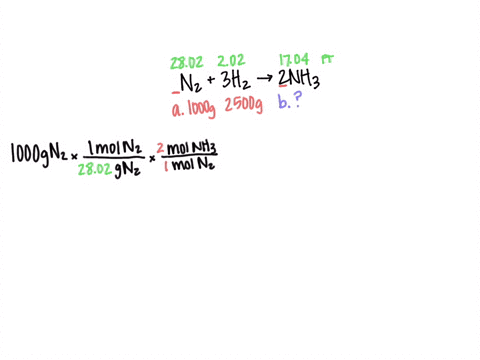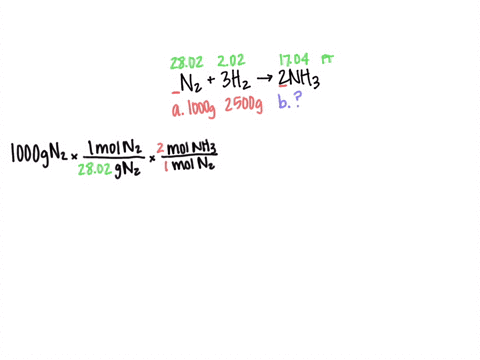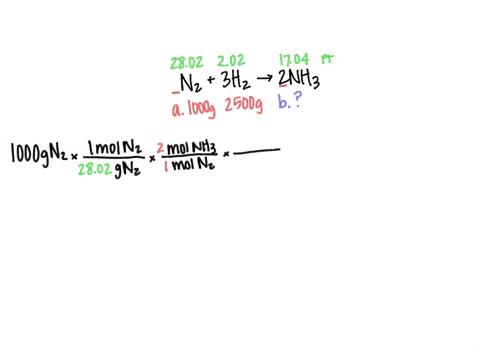
SOLVED: Al2(SO4)3(aq) + K2SO4(aq) + 24 H2O(liq) → 2 KAl(SO4)2•12 H2O 2 KAl(OH)4 (aq) + H2SO4 (aq) →2 Al(OH)3 (s) + K2 SO4 (aq) + 2 H2O(l) 2 Al(OH)3 (s) + 3H2SO4 (

SOLVED: There are several steps to the synthesis of the aluminum potassium alum that can be represented by the overall reaction.2Al(s) + 2KOH(aq) + 4H2SO4(aq) + 24H2O(l) → 2KAl(SO4)212H2O(s) + 3H2(g) +H2O(l)1.
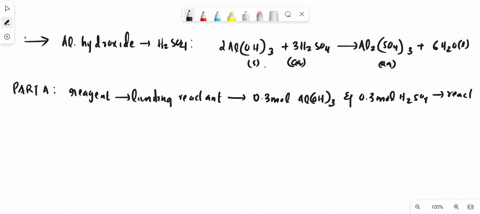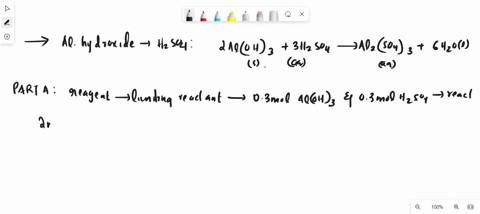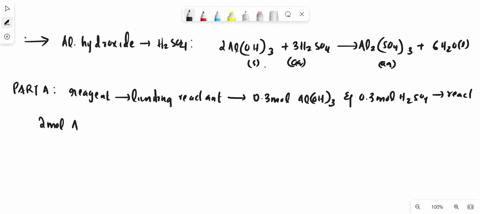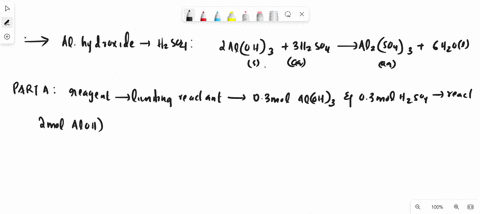
SOLVED: Al2(SO4)3(aq) + K2SO4(aq) + 24 H2O(liq) → 2 KAl(SO4)2•12 H2O 2 KAl(OH)4 (aq) + H2SO4 (aq) →2 Al(OH)3 (s) + K2 SO4 (aq) + 2 H2O(l) 2 Al(OH)3 (s) + 3H2SO4 (

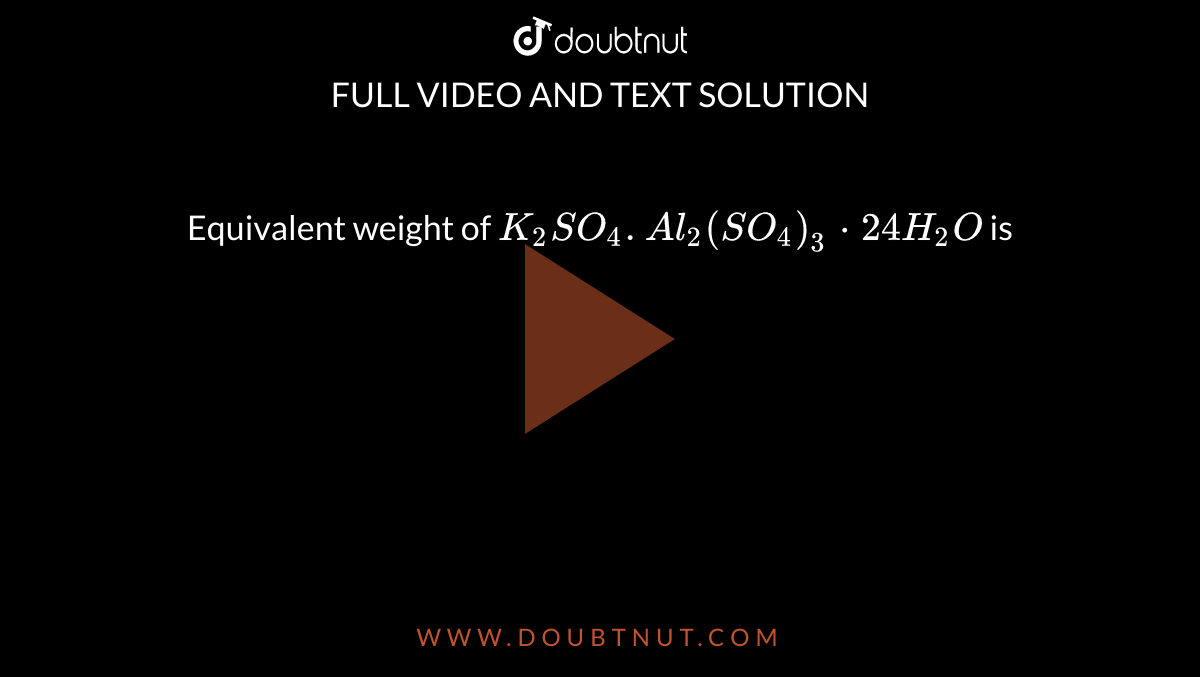
The equivalent weight of k2so4 Al2(so4)3 24H2O - Chemistry - Some Basic Concepts of Chemistry - 13624791 | Meritnation.com

Stoichiometry: Preparation of Aluminum - Lab Experiment 4 | CHE 201 | Study notes Chemistry | Docsity

3%20+%20K2SO4%20+%20H2O%20=%20KAl(SO4)2*12H2O.svg)