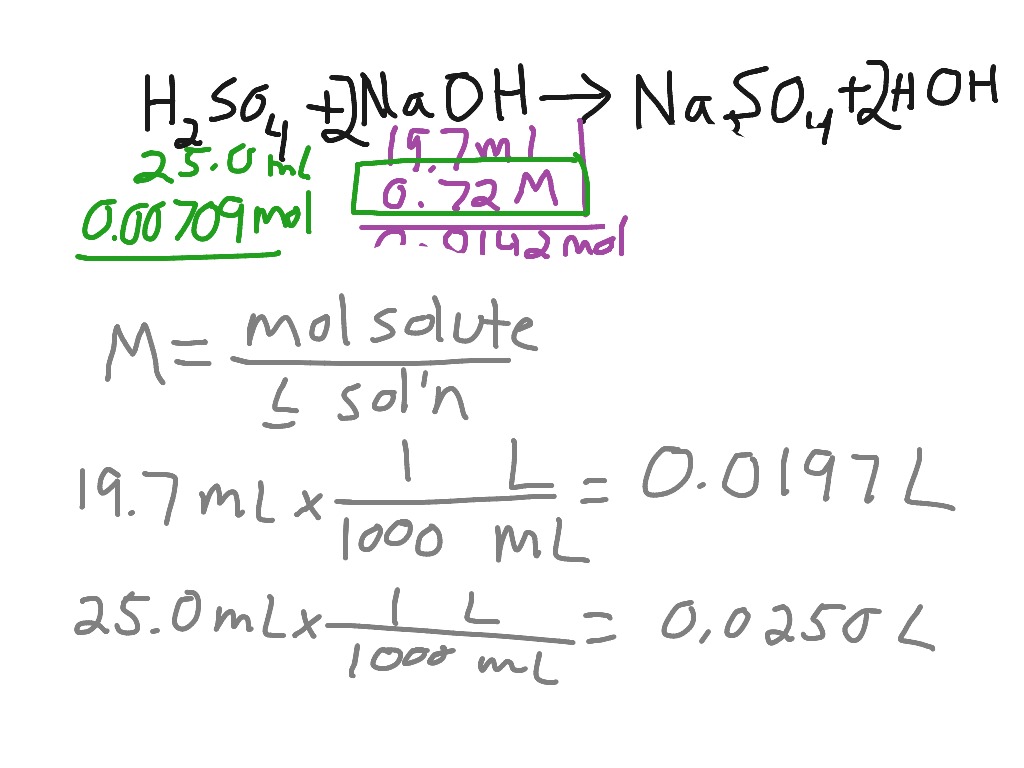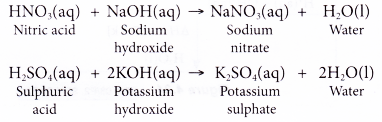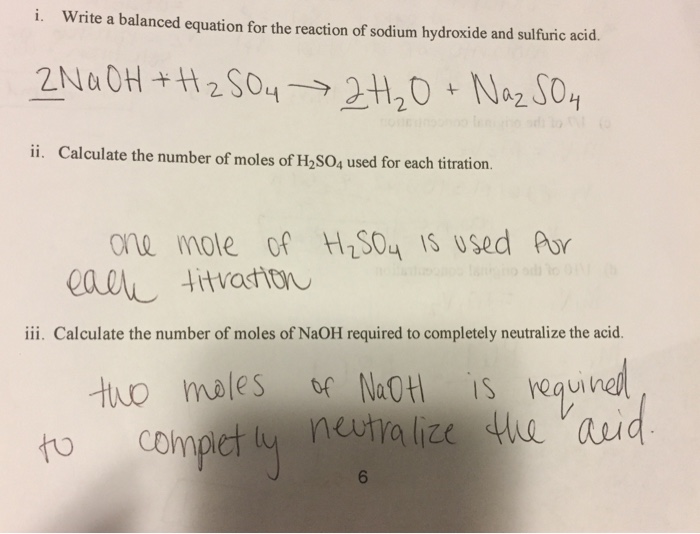KR100847444B1 - Method for neutralizing sulphuric acid waste water by using calcined dolomite - Google Patents

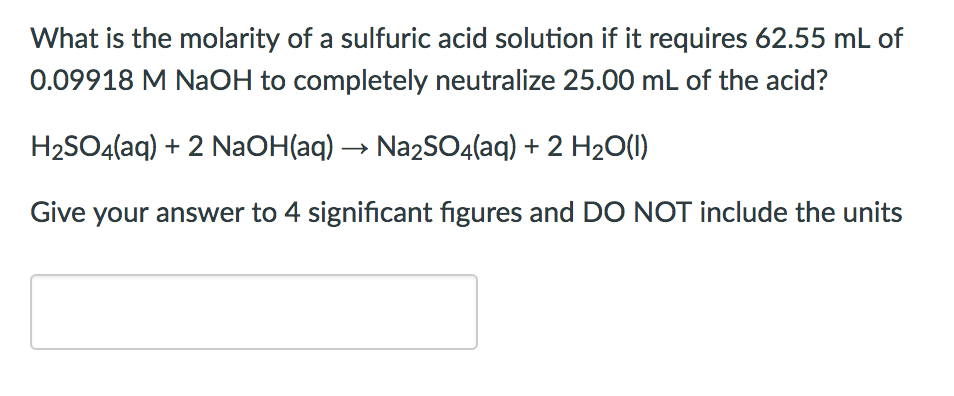
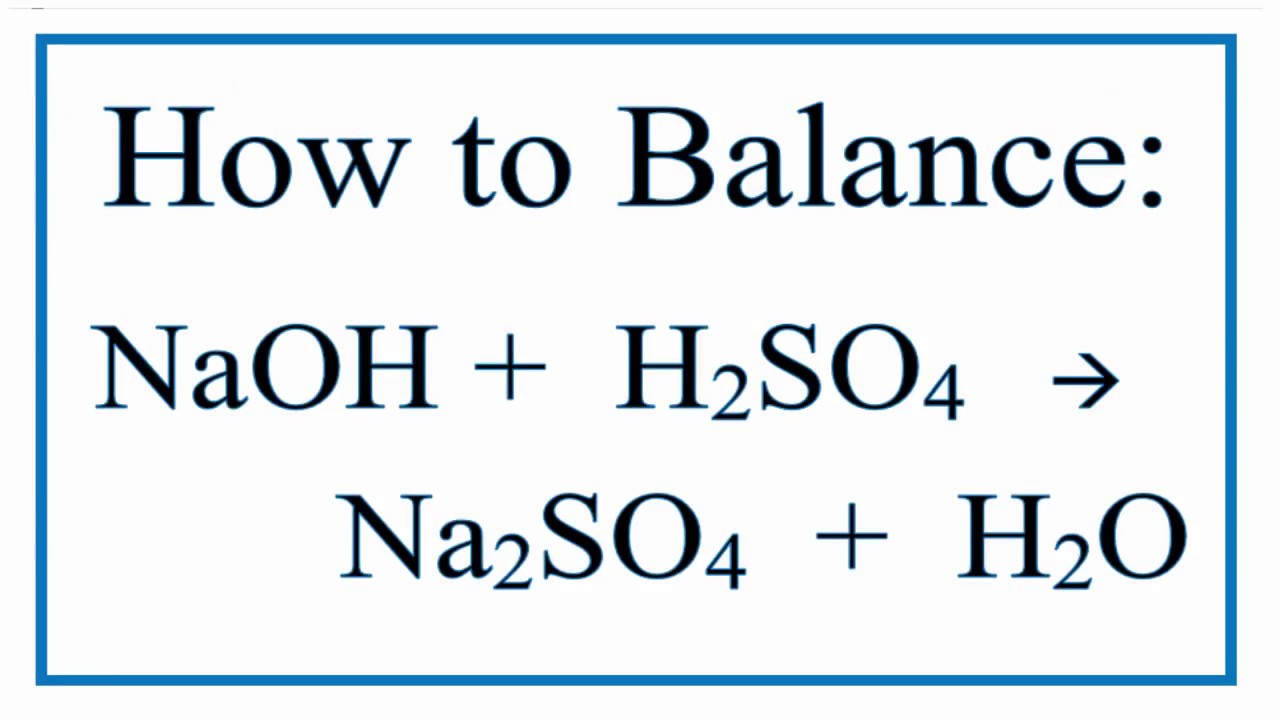
SOLVED: Assignment 3 In a titration of sulfuric acid against sodium hydroxide, 32.20mL of0.250 M NaOH is required to neutralize 26.60mL of H,SOA Calculate the molarity of the sulfuric acid. E,SO, (aq) +

Neutralization Reaction: Determine Molarity of a sulfuric Acid Solution when Neutralized by NaOH - YouTube
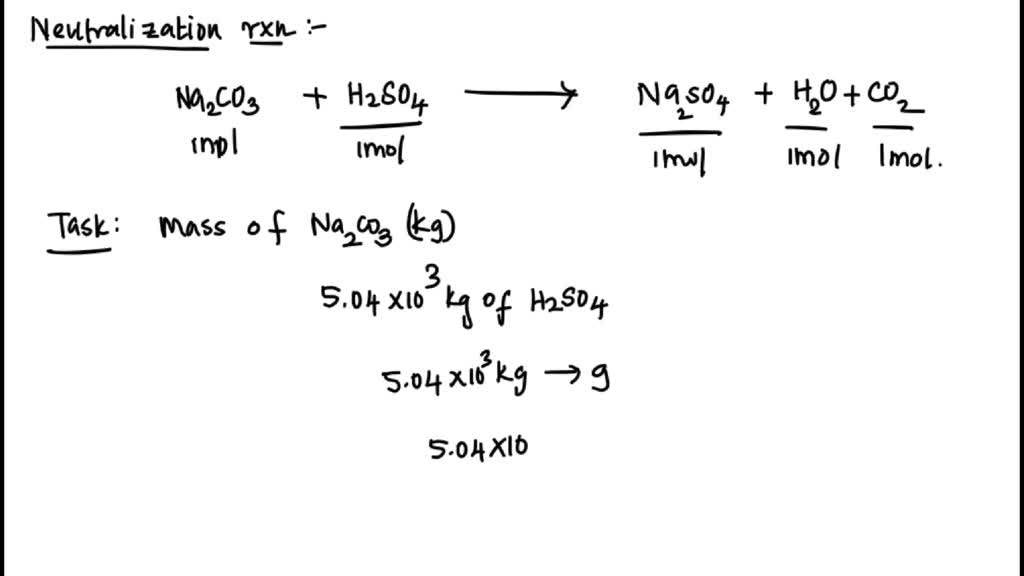
SOLVED: Sodium carbonate (Na2CO3Na2CO3) is used to neutralize the sulfuric acid spill. How many kilograms of sodium carbonate must be added to neutralize 5.04×103 kgkg of sulfuric acid solution?

Neutralization curve of Mg(OH)2 (MH) with a sulfuric acid solution (0.4 M). | Download Scientific Diagram

WARM UP 1. Write the equation for the neutralization reaction between sulfuric acid (H 2 SO 4 ) and ammonium hydroxide (NH 4 OH). - ppt download

SOLVED: Determine the volume of 0.240 M KOH solution required to neutralize each sample of sulfuric acid. The neutralization reaction is: H2SO4(aq)+2KOH(aq)→ K2SO4(aq)+2H2O(l) 40 mLmL of 0.240 MM H2SO4H2SO4 Express your answer

Question Video: Calculating the Volume of Sulfuric Acid That Completely Neutralizes a Given Volume and Concentration of Sodium Hydroxide | Nagwa