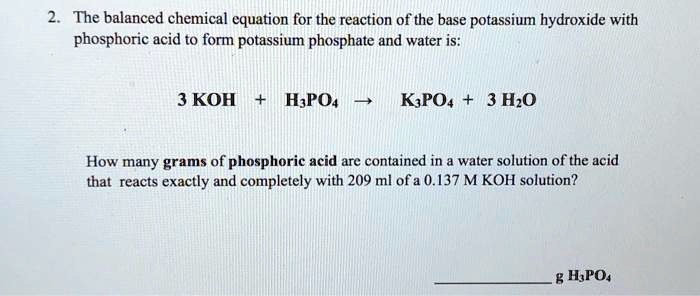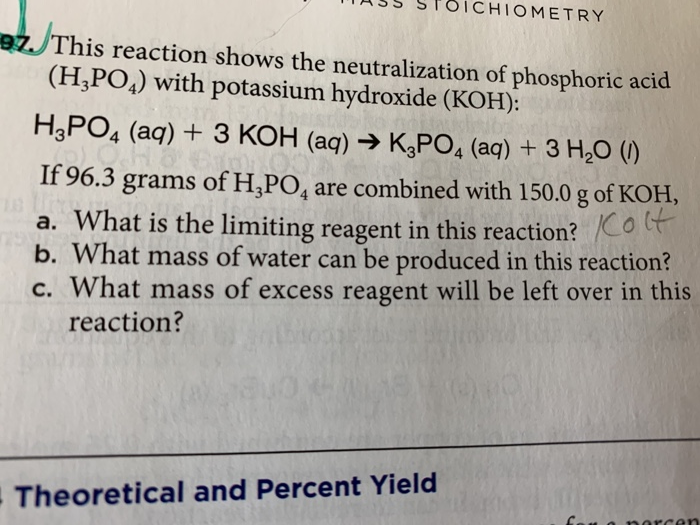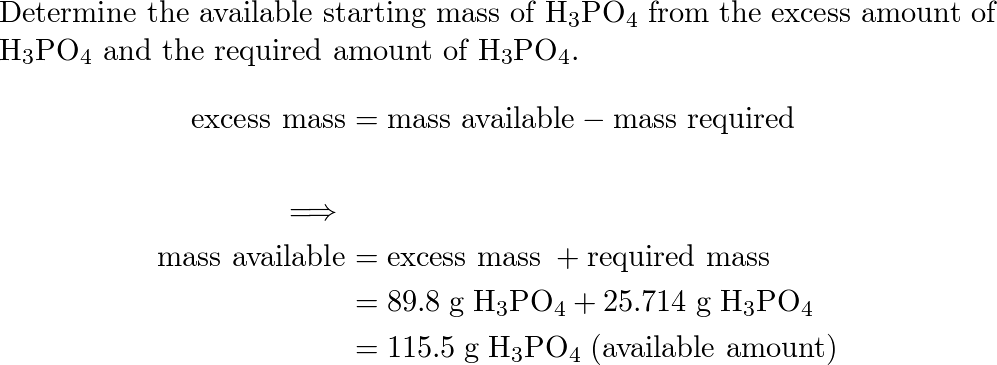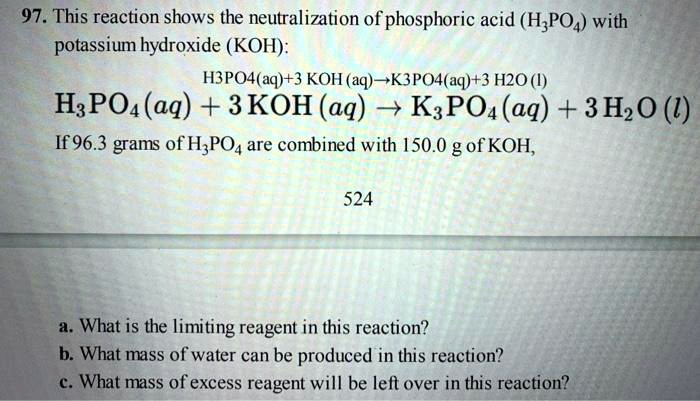
SOLVED: 97. This reaction shows the neutralization of phosphoric acid (H;POA) with potassium hydroxide (KOH): H3PO4(aq)+3 KOH (aq)K3PO4(aq+3 H2O (4) HzPOa (aq) + 3KOH (aq) = K:POa (aq) + 3Hz0 (U) If

H3PO4+KOH=K3PO4+H2O Balanced Equation||Phosphoric acid+Potassium hydroxide=Potassium phosphate+Water - YouTube
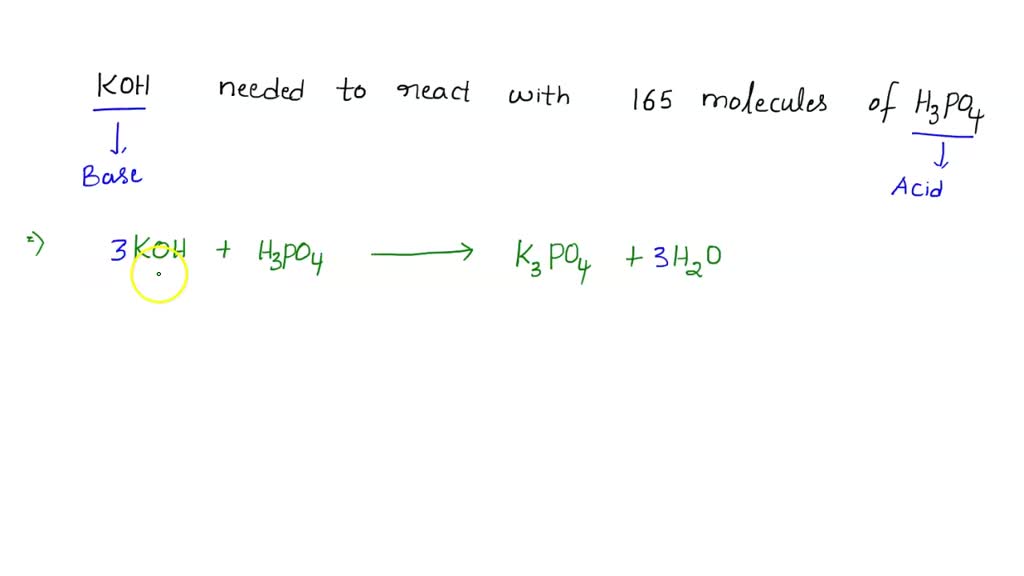
SOLVED: 'How many units of potassium hydroxide are needed to react with 165 molecules of phosphoric acid? BKOHq) HzPO4aq) KzPO4aq) 3HzQ()'
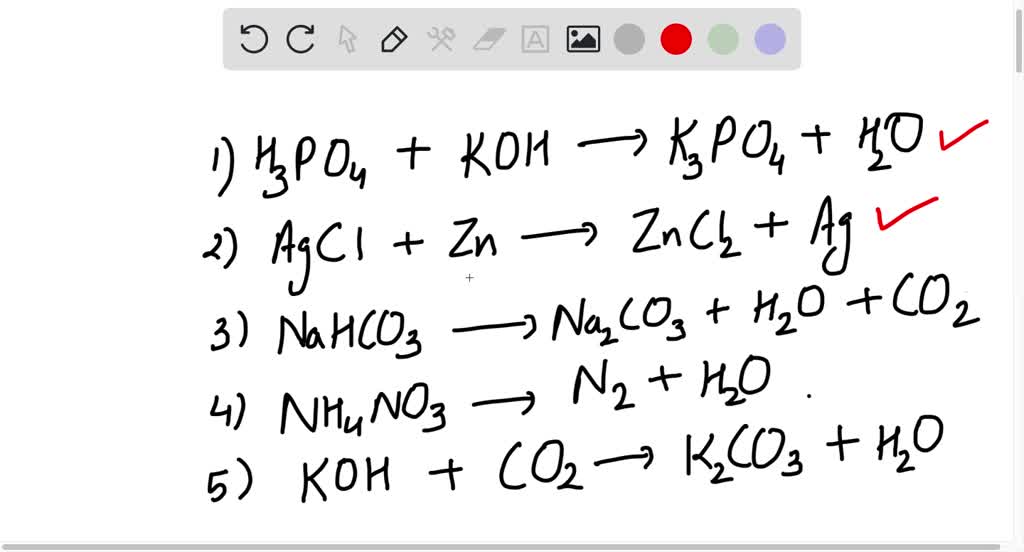
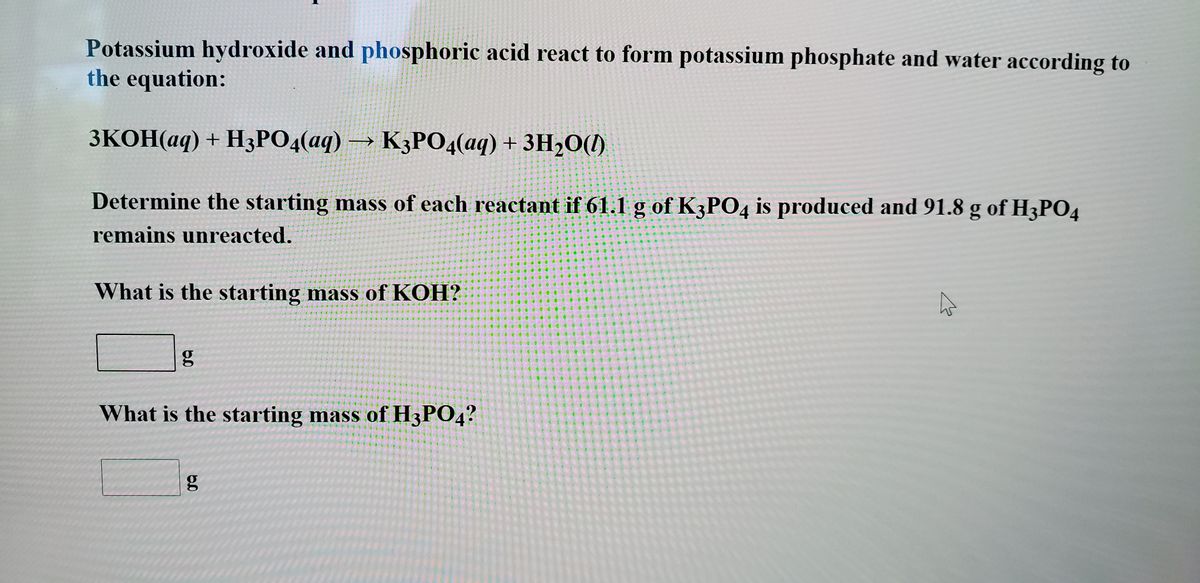
SOLVED: Write an unbalanced equation to represent each of the following reactions: (a) Potassium hydroxide and phosphoric acid react to form potassium phosphate and water. (b) Zinc and silver chloride react to
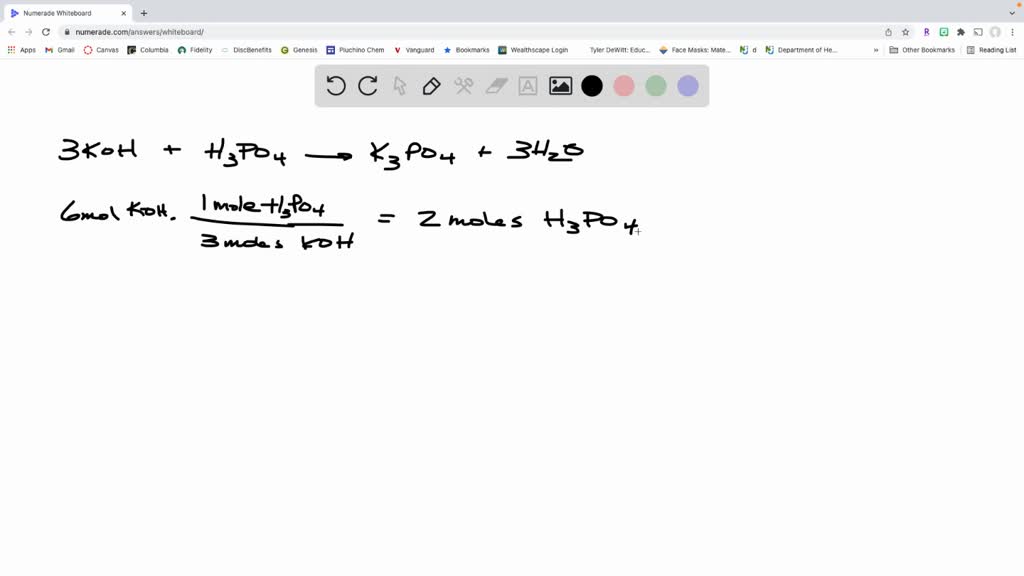
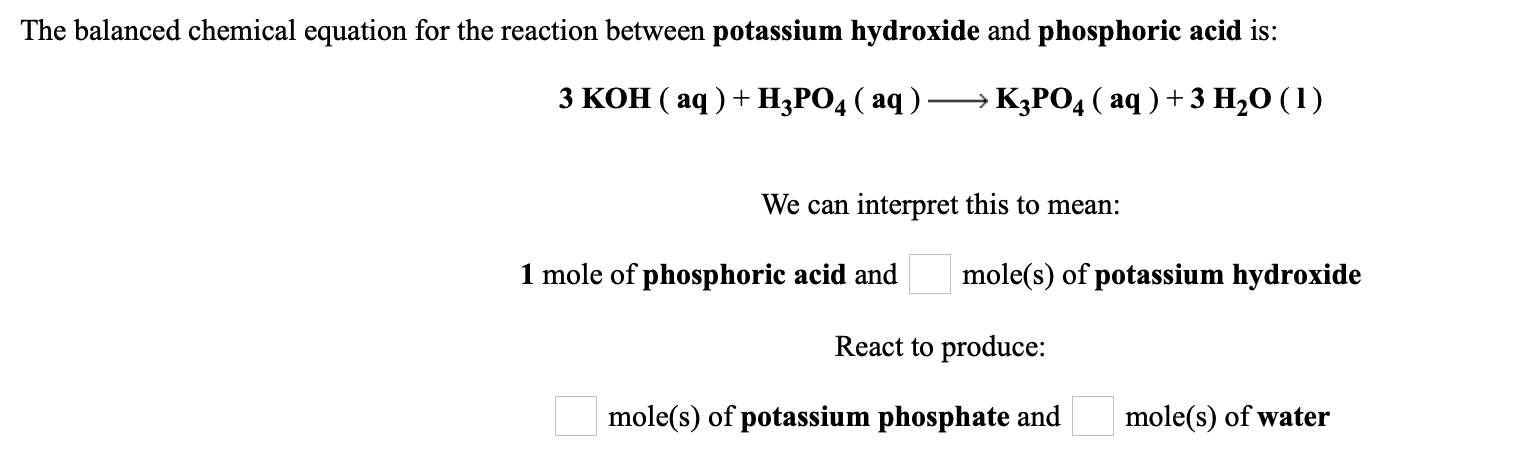
SOLVED: When potassium hydroxide reacts with phosphoric acid, potassium phosphate and water are produced. The balanced equation for this reaction is: 3KOH(aq) + H3PO4 (aq) -> K3PO4(aq) + 3H2O(l) If 6 moles

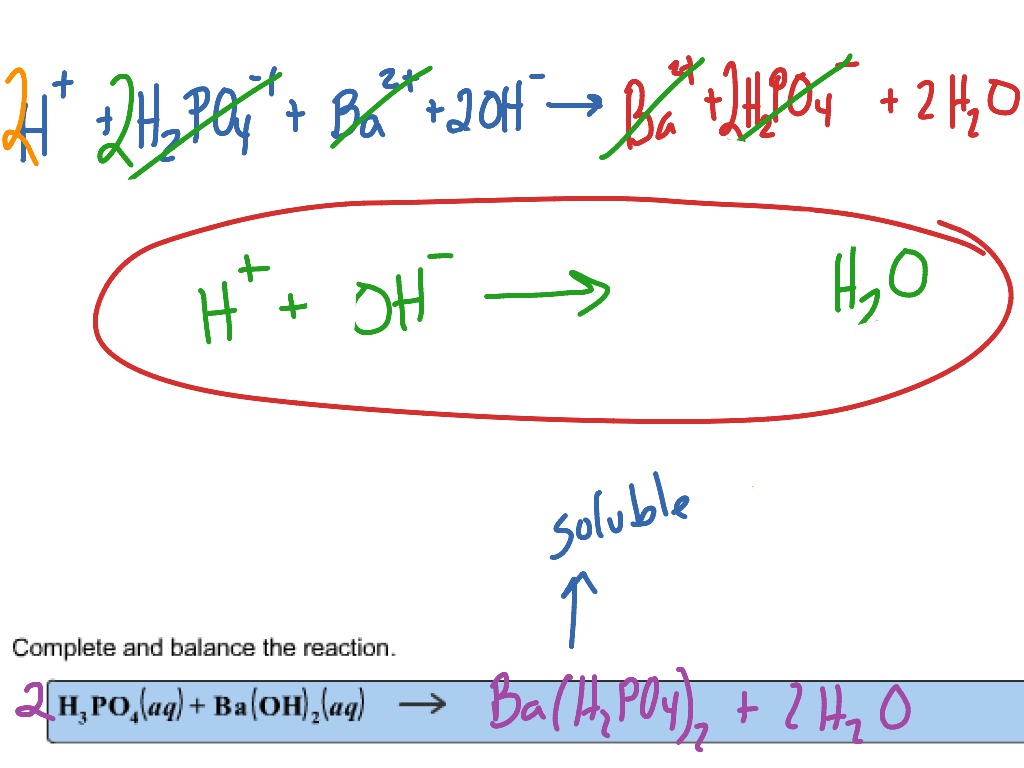
Outline of AC preparation from RH. In figure, KOH, ZnCl2, H3PO4, and N... | Download Scientific Diagram
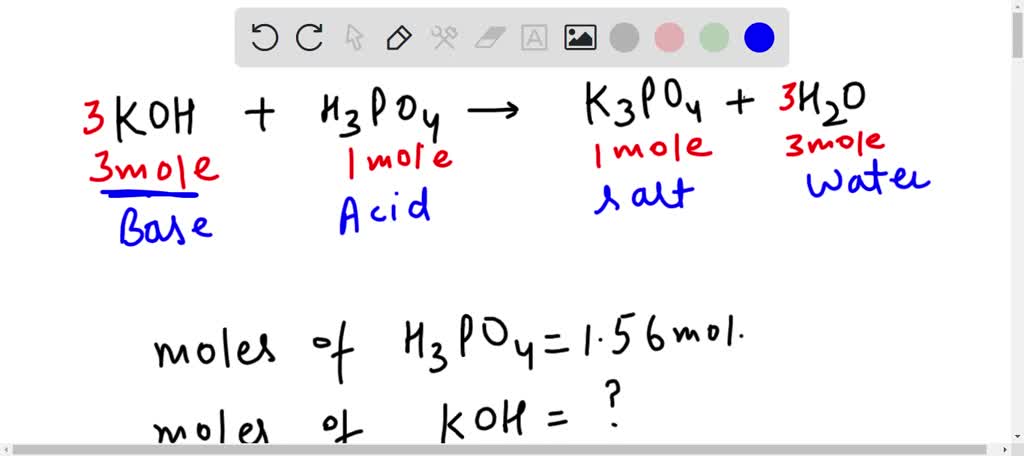
SOLVED: How many moles of potassium hydroxide (KOH) are needed to completely neutralize 1.56 mol phosphoric acid (H3PO4)?

Ch19.1 – Acids and Bases Acids - corrosive, taste sour, put electrolytes in soln, react with metals Ex1) Single displacement reaction: H 2 SO 4(aq) + Zn. - ppt download
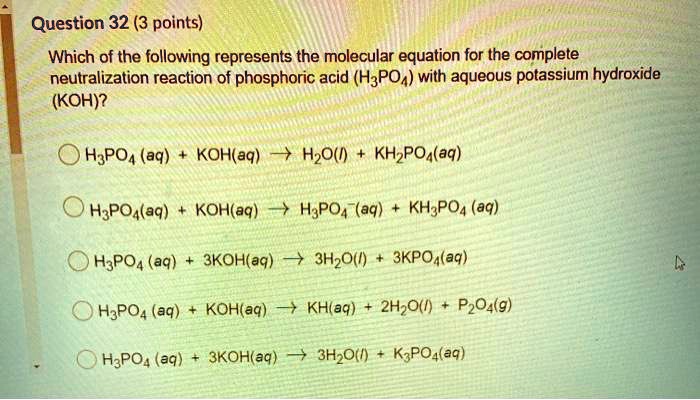
SOLVED: Question 32 (3 points) Which of the following represents the molecular equation for the complete neutralization reaction of phosphoric acid (HzPOA) with aqueous potassium hydroxide (KOH)? HyPOa (aq) KOH(aq) Hzo() KHzPOA(aq)
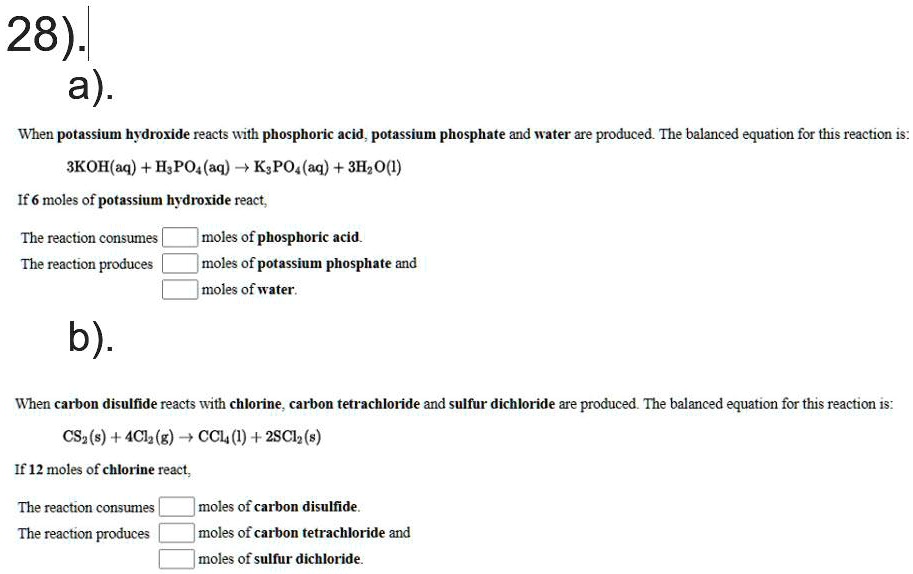
SOLVED: 28)| a) When potassium hydroxide reacts With phosphoric acid potassium phosphate and water are produced The balanced equation for this reaction is 3KOH(aq) + H;PO (aq) K;POA (aq) + 3H20() If

OneClass: 2.For the reaction of phosphorous acid (H3PO3) and potassium hydroxide (KOH), write (a) the...
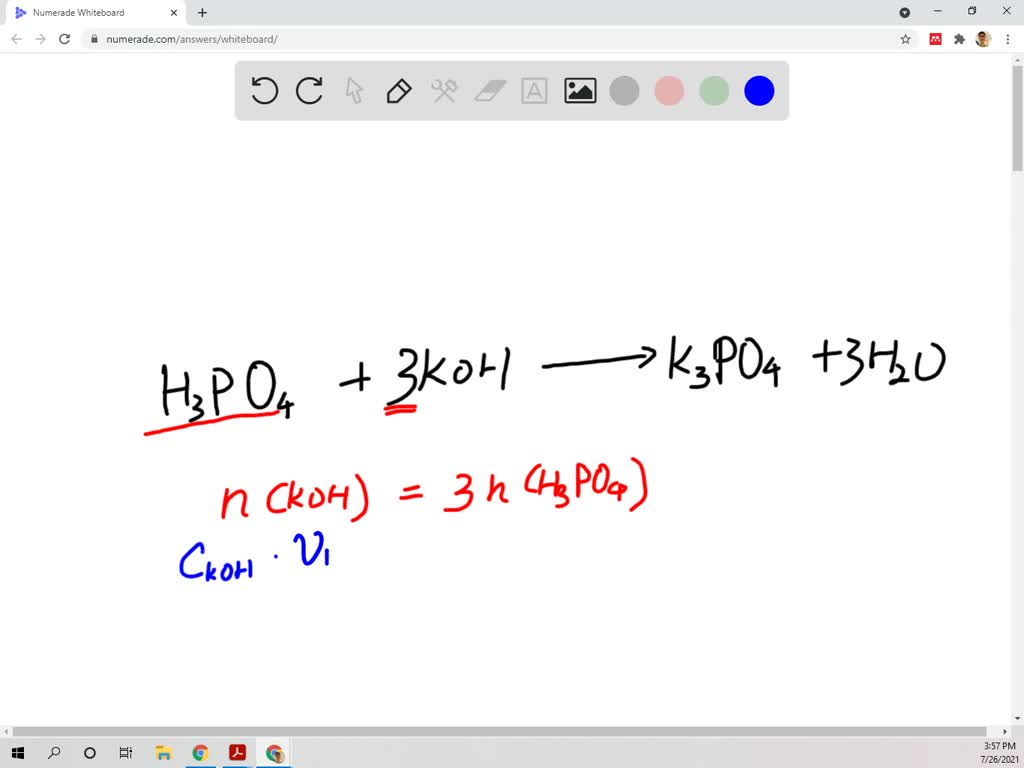
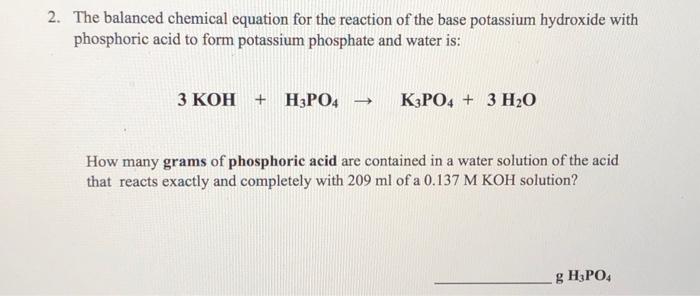
SOLVED: A 0.0700 L sample of phosphoric acid (H3PO4) solution with an unknown concentration reacts with 0.200 L of 0.300 M potassium hydroxide ( KOH) solution. What is the concentration of the phosphoric