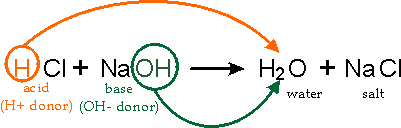
Aqueous hydrochloric acid HCl will react with solid sodium hydroxide NaOH to produce aqueous sodium - Brainly.com
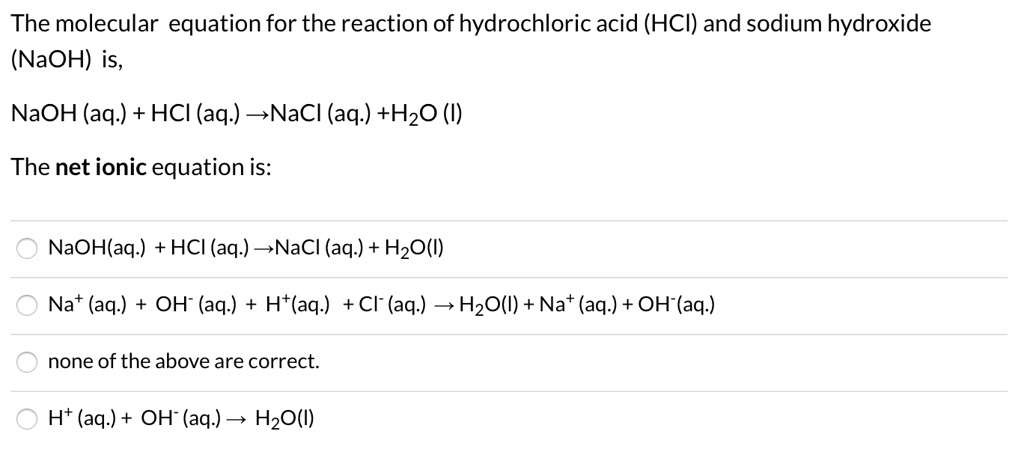
SOLVED: The molecular equation for the reaction of hydrochloric acid (HCI) and sodium hydroxide (NaOH) is, NaOH (aq ) + HCI (aq-) NaCI (aq ) +HzO (I) The net ionic equation is:

Draw the products of benzoic acid reacting with sodium hydroxide. Draw the products of the pyridine reacting with hydrochloric acid. Use the "+/-" button to add the charge (and H atom).
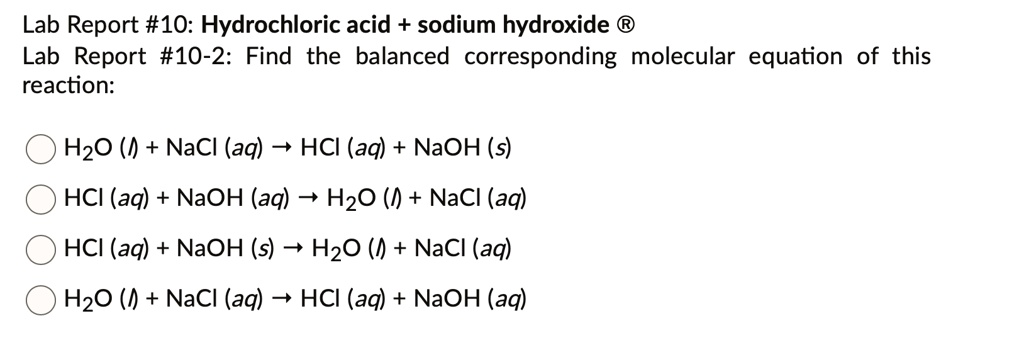
SOLVED: Lab Report #10: Hydrochloric acid + sodium hydroxide Lab Report #10-2: Find the balanced corresponding molecular equation of this reaction: H2O (0 + NaCl (aq) 4 HCI (aq) + NaOH (s)
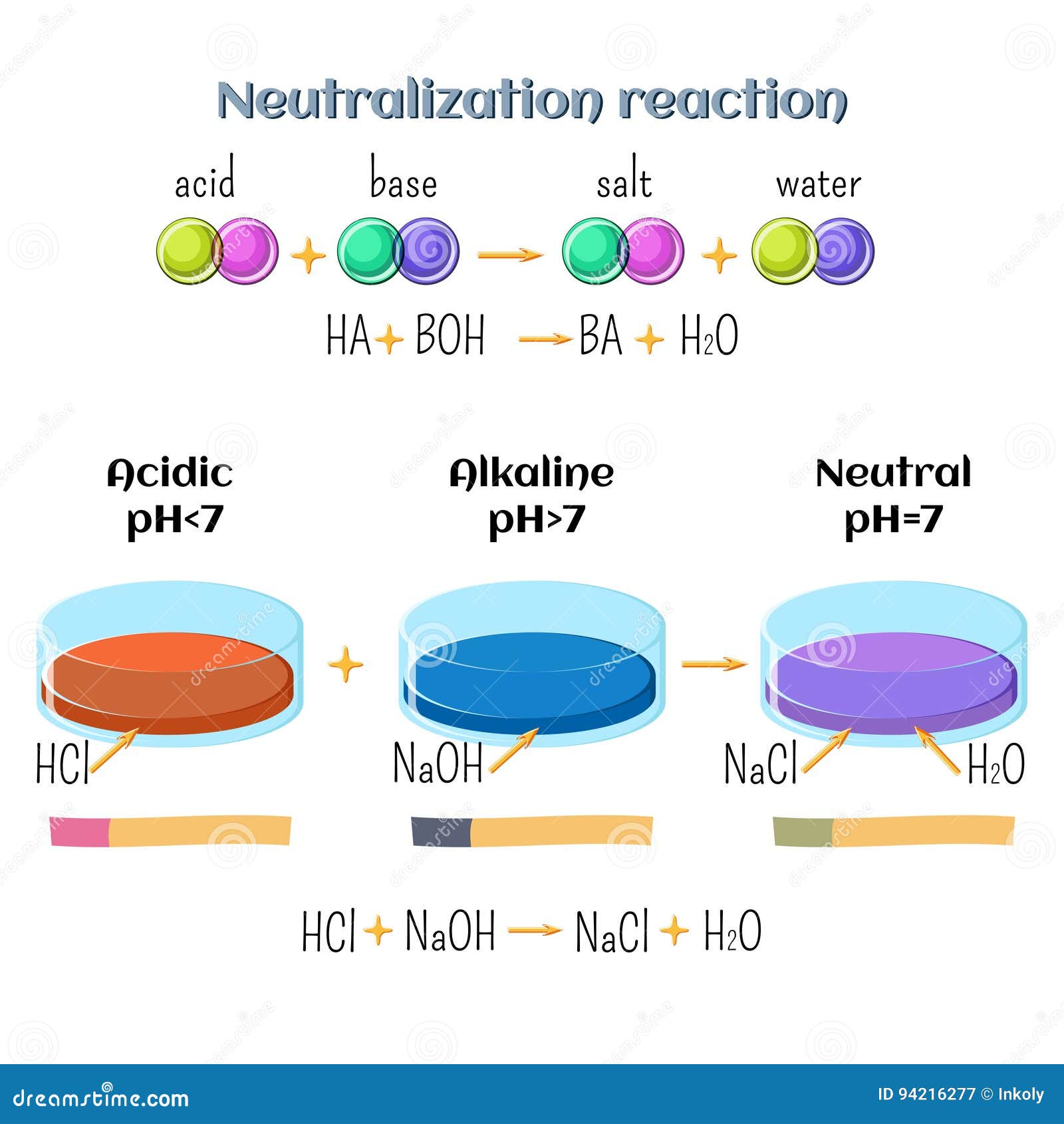
Acid-base, Neutralization Reaction of Hydrochloric Acid and Sodium Hydroxide. Types of Chemical Reactions, Part 6 of 7 Stock Vector - Illustration of acid, atom: 94216277

Write the neutralization reaction between Hydrochloric acid HCI and sodium hydroxide NaOH, and write the equation for this process.

Ionic equations A chemical equation shows the number of atoms and molecules of the reactants and products. Also shows physical state of reactants and products. - ppt download



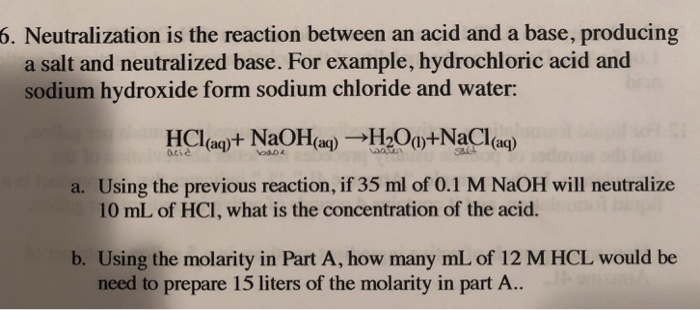
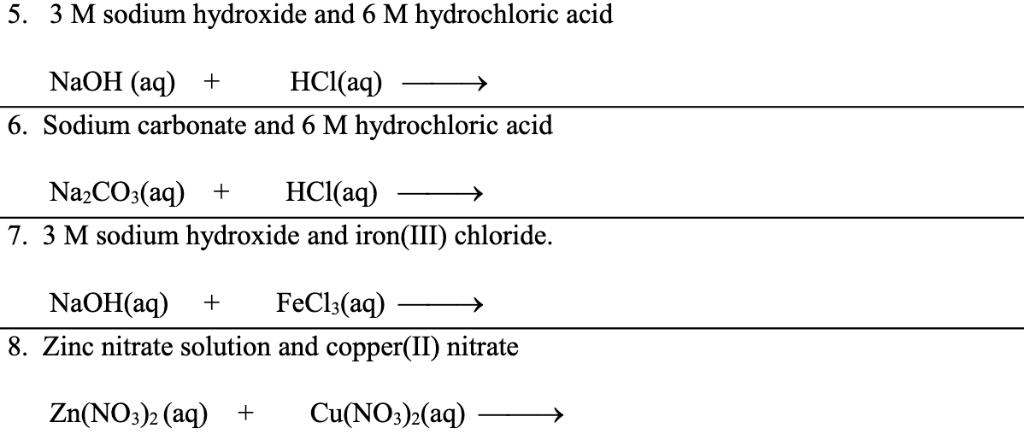




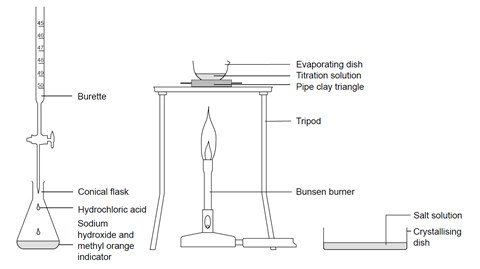


![ANSWERED] Write a balanced chemical equation for t... - Inorganic Chemistry ANSWERED] Write a balanced chemical equation for t... - Inorganic Chemistry](https://media.kunduz.com/media/sug-question/raw/61174058-1657039371.6671314.jpeg)