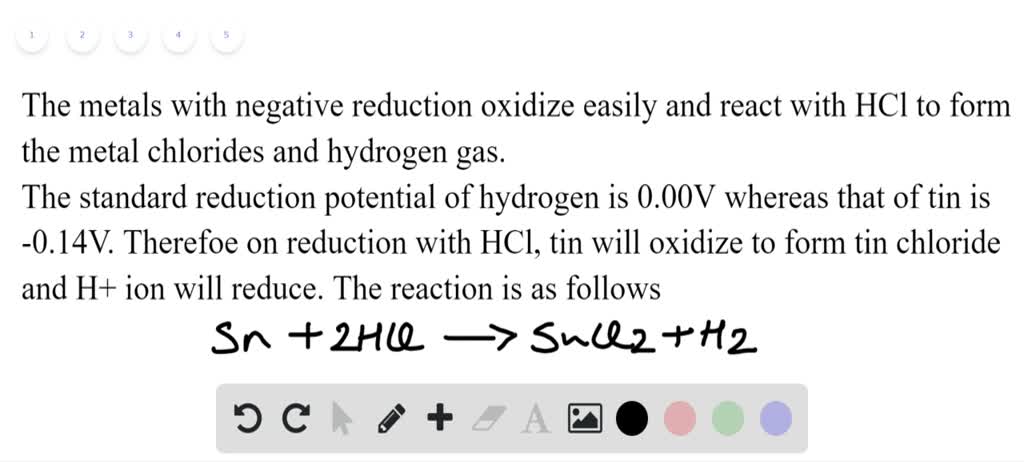
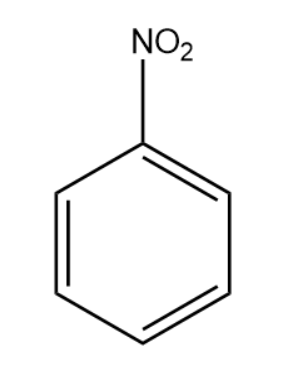
Answer in one sentence. Predict the product of the following reaction. Nitrobenzene→Sn/conc⋅HCl? - Chemistry | Shaalaa.com

Reaction of x g of Sn with HCl quantitatively produced a salt. Entire amount of the salt reacted with y g ofnitrobenzene in the presence of required amount of HCl to produce

reaction mechanism - What is the graphical formula when nitrobenzene is added to HCL and tin? - Chemistry Stack Exchange

Effect of initial HCl concentration on the stripping of indium and tin... | Download Scientific Diagram

Leaching of tin from waste Pb-free solder in hydrochloric acid solution with stannic chloride - ScienceDirect

a) Draw the mechanism for the reaction of tin/HCl with m-nitroacetophenone. b) Why is sodium hydroxide added to the reaction? What tin compounds are produced? How do you separate the tin salts